The Core Principle of Quantum Sensing
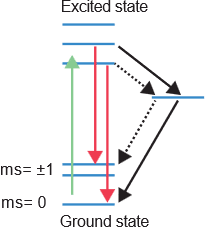
Quantum sensing of free radicals relies on the precise excitation of electrons. When photons of appropriate wavelengths interact with specific electrons within our sensors, they excite these electrons from their ground state to an excited state. As these excited electrons naturally decay back to their ground state, they release photons, which we can detect. These emitted photons typically exhibit a red shift compared to the initial excitation wavelengths (as conceptually shown in Figure 1). The rate at which these photons are emitted and the time it takes for the excited electrons to relax back to their ground state (known as T1 relaxation time) are profoundly influenced by the local magnetic environment. Stronger magnetic interference around an electron leads to a shorter T1 decay time. This fundamental principle forms the bedrock of our quantum sensing approach.
Unlock previously inaccessible samples
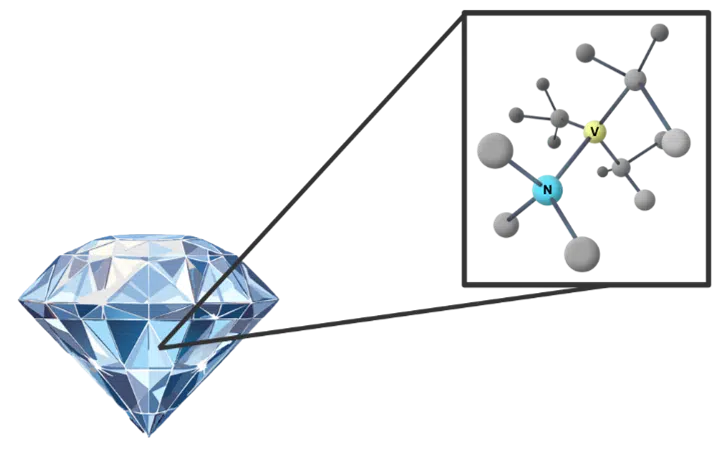
To precisely measure these subtle magnetic interferences, we employ highly sensitive quantum sensors built into Fluorescent Nanodiamonds (FNDs). These FNDs contain Nitrogen-Vacancy (NV) centers within their diamond lattice (see Figure 2 for a representation). An NV center is ingeniously created by removing a carbon atom using an electron beam and replacing it with a nitrogen atom. This atomic manipulation leaves a vacancy next to the nitrogen atom, which harbors unbound electrons. These vacancy electrons can be excited by laser light (typically in the 500-600 nm range) and, upon decay, emit light in a distinct red-shifted range (650-800 nm). The unique quantum properties of these NV centers make them exquisitely sensitive to minute magnetic fields, allowing them to function as ideal nanoscale probes.
Free Radical Detection by Nanodiamonds
The magic of detecting free radicals lies in their unique property: they possess an unpaired electron. This unpaired electron carries a distinct magnetic dipole moment, creating a localized magnetic interference in its direct vicinity. The electrons within an NV center are exquisitely sensitive to this magnetic moment. When a free radical comes near an NV center, its magnetic field subtly alters the NV center’s fluorescence properties and, crucially, its T1 relaxation time. By measuring this change, we can precisely quantify the presence and concentration of free radicals. This method is highly specific, targeting the unpaired electron spin, allowing us to differentiate free radicals (like hyperactive superoxide and hydroxyl radicals) from non-radical Reactive Oxygen Species (ROS) such as hydrogen peroxide. High concentrations of oxygen free radicals are known to cause oxidative stress, damaging DNA, RNA, proteins, and lipids, ultimately impacting cell health.
Quantum Nuova: Real-Time, Subcellular Insights
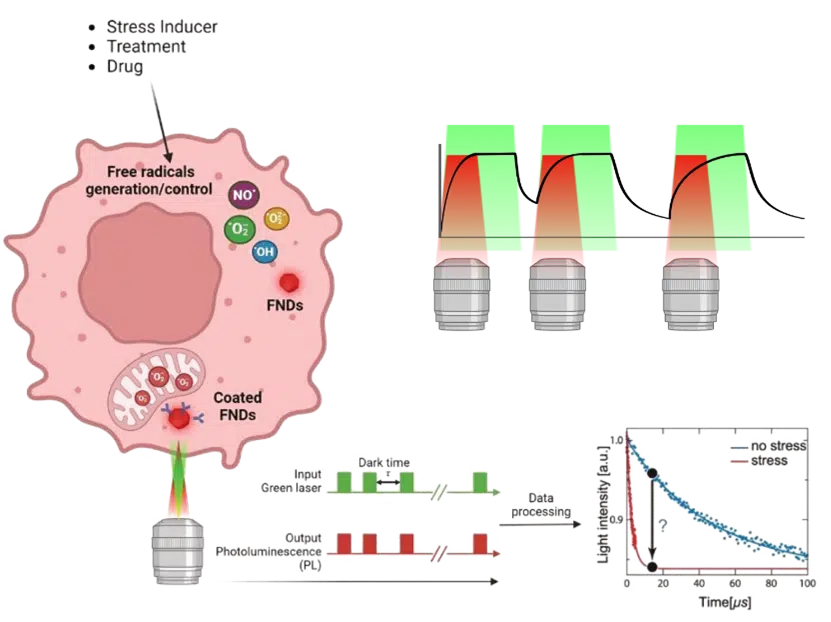
Our Quantum Nuova system leverages these nanodiamonds to revolutionize free radical detection. These FNDs are biocompatible and can be readily introduced into cells, often absorbed via endocytosis. Once inside, our Quantum Nuova platform, using a modified confocal microscopy system, allows us to obtain real-time measurements of free radical concentration with exceptional spatial resolution, down to approximately 50nm around each nanodiamond. This subcellular precision is key. Furthermore, these nanodiamonds can be functionalized (e.g., with antibodies, biotin, or PEG) to specifically target organelles like mitochondria, enabling accurate readouts of free radical production and neutralization in precise cellular locations.
To precisely determine the free radical concentration, Quantum Nuova utilizes the T1 relaxometry method, correlating the NV center’s photon emission time with the intervals between laser excitations (as illustrated in Figure 3). The system applies laser pulses with increasing intervals, monitoring the initial re-excitation to determine the photon emission rate. The dark time (time between pulses) and emitted photons are then plotted to create a relaxation curve. By fitting a specific mathematical model to this curve, the T1 relaxation time can be accurately determined. The shorter the T1 time, the higher the local free radical concentration. This method empowers a new avenue to explore free radicals in single cells, allowing researchers to discern differences in free radical levels between dysfunctional and normal cells and correlate these findings directly to disease phenomena.
At QT Sense, we enable the detection of free radicals in single cells with unprecedented sensitivity, precision, and temporal resolution, opening up entirely new possibilities in redox biology and beyond.
Share this:





